Chapter:
Suppose water rise predicted by capillarity formula exceeds height of capillary tube. Does water overflow? Explain with mamatical expression.
We know that Capillary action occurs when the force of adhesion between molecules of liquid and walls of a capillary tube (container) is stronger than the force of cohesion between molecules of a liquid. Due to this the liquid has the ability to flow in narrow spaces, or even against gravity.
The height
h of a liquid column is given as
`h=(4 sigma cos theta)/(rho g d)`
where
`sigma` is the liquid-air surface tension.
`theta` is angle of contact,
`rho` is the density of liquid,
`g` is acceleration due to gravity, and
`d` is the diameter of capillary tube.
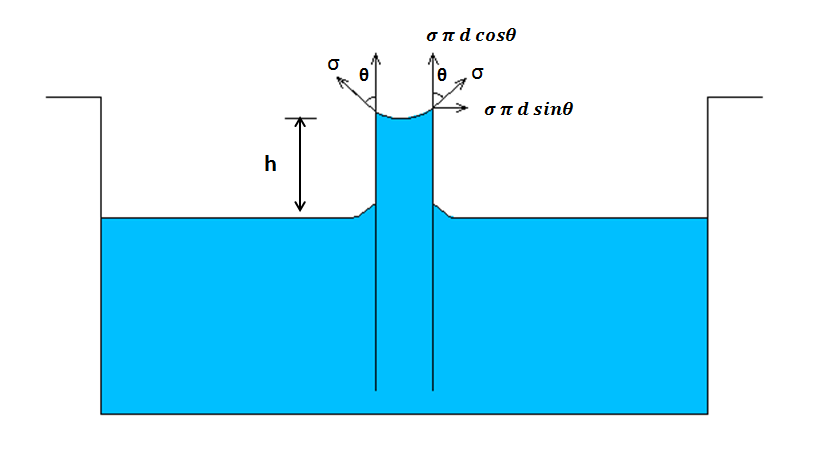
When length of tube is less than the height of cap....
Show MoreAll Chapters
Dimensional Analysis, Similitude and Physical Modelling
Flow Past Through Submerged Body
Boundary Layer Theory
Properties of fluid
- properties of fluids
- complete IOE solution on fluid mechanics
- Detailed description chapterwise
- Unlimited Numericals problem solved with updation daily
- And much more
Hydrostatics
- Hydrostatics basic theory
- All important Numericals
- IOE,PU, MIT,A.M.I.E,Delhi university,EUPS,Engineering service exam solved problems
- Detailed explanation
Hydrostatics- Buoyancy and metacentric height
- detailed explanation of force of buoyancy, metacentric height,stability of floating body
- unlimited numericals solved with updation weekly
- including IOE , PU, KU, GATE, MIT, ANNA UNIVERSITY,UPSC EXAM SOLUTIONS
- And much more
 Guest
Guest