Chapter:
1.
Soil structure is usually defined as the arrangement and state of aggregation of soil particles is a soil mass. Soil structure is an important factor which influences many soil properties, such as Permeability, compressibility and shear strength etc. The following types of soil structure are generally recognised:
- Single grained
- Honeycomb
- Flocculent
- Dispersed
- Coarse-grained skeleton
- Cohesive Matrix
Single Grained:
An arrangement composed of individual soil particles.This type of structure is found in case of coarse grained soil deposits. The major forces causing their deposition is gravitational forces and the surface forces are too small to produce any effect. There will be particles to particles contact on the deposit.
Honeycomb:
It is an arrangement of soil particles having a comparatively loose, stable structure resembling a honeycomb.
This type of structure is associated with silt deposits. The major forces causing their deposition are gravitational force and surface forces.
Soil with Honeycomb structure are loose and have high void ratio. They can support loads only under static conditions. Under vibration and shocks, the structure collapse and large deformation takes place.
Flocculent:
An arrangement composed of
Flocculated structures generally occurs in clay. In case of flocculated structure, there will be edge to edge and edge to face contact between the particles. This type of formation is due to the net electrical forces(attractive) between the adjacent particles at the time of deposition.
Soil with flocculated structure are light in weight and have very high void ratio and water content. However, these soils are quite strong and can resist external forces because of strong bond due to attraction between the particles.
Dispersed:
Dispersed structure occurs in remoulded clay. The particles develop more or less parallel orientation. When clay soil are transported from one place to another place by nature or man and get remoulded, dispersed structure develops in clay. Remoulding converts the edge to face orientation to edge to edge orientation. The dispersed structure is formed in nature when there is a net repulsive force between particles.
The soil in dispersed structure have high compressibility, low shear strength and low permeability.
Coarse-grained skeleton:
A coarse grained skeleton is a composite structure which is formed when the soil contains particles of different types. When the amount of bulky, cohesion-less particles is large compared with that of fine grained clayey particles, the bulky grains are in particle to particle contact. These particles forms a skeleton.
As long as the soil structure is not disturbed, acparse grained skeleton can take heavy loads without much deformation.
Cohesive matrix(clay matrix) structure:
It is an arrangement in which a particle-to-particle contact of coarse fraction is not possible.
Clay-matrix structure is also a composite structure formed by soils of different types. However, in this case, the amount of clay particles is very large compared to bulky coarse grained particles. The clay forms a matrix in which bulky grains appear floating without touching one another.
2. Explain structures of various Clay Minerals.
The structures of a few important clay minerals are described below:
Kaolinite:
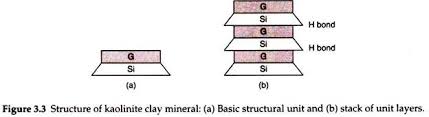
The kaolinite is the most common mineral of the kaolinite group of minerals. Its basic structural unit consists of an aluminium sheet (gibbsite G) combined with silica sheet S. Tips of the silica sheet and one base of the Alumina sheet from a common interface. The total thickness of the structural unit is about 7 Angstorm (`A^0`). The kaolinite mineral is formed by stacking, one over the other, several such basic structural units. Figure below shows two such units.
The structural units join together by hydrogen bond, which develops between the oxygen of silica acid and hydroxyl of aluminium sheet. As the bond is fairly strong, the mineral is stable. Moreover, water cannot easily enter between the structural units and causes expansion.
The Kaolinite mineral is electrically neutral. However in the presence of water, some hydroxyl ion dissociate and loose hydrogen and leave the Crystal with a small residual negative charge . The flat surface of the mineral attract positive ions (cation) and water. A thick layer of absorbed water is formed on the surface
The Kaolinite mineral generally have a hexagonal shape in plan, with the side of the hexagon between 0.5 to 1.0 Micron. The thickness of the mineral is about 0.05 Micron. The specific surface is about `15 M^2/g`. The most common example of the kaolinite mineral is China clay.
Halloysite is a clay mineral which has the same basic structure is kaolinite, but in which the successive structural units are more randomly packed, and are separated by a single molecular layer of water. The properties of halloysite depend upon this water layer. If the water layer is removed by drying, the properties of the mineral drastically changes.Halloysite particles are tabular in shape, in contrast to the the platy shape of kaolinite particle. The soils containing halloysite have a very low mass density.
Commercial Uses of Kaoline:
Kaolin is used in ceramics, medicine, coated paper, cosmetics and as a food additive in toothpaste, and also as a light diffusing material in white incandescent electric bulbs. It is generally the main component in porcelain.
It is also used as filler for paint, rubber, and plastics as it is relatively inert and is long lasting. However, the greatest demand for kaolinite is in the paper industry to produce a glossy paper. Natural kaolinite usually contains small amounts of uranium and thorium. Hence, it is useful in radiological dating.
Montmorillonite:
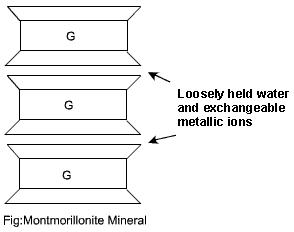
The Montmorillonite is the most common mineral of the Montmorillonite group of minerals. The basic structural unit consists of an aluminium sheet sandwiched between two silica sheets. Successive structural units are stacked on over another, like leaves of a book. Figure below shows two such structural unit. The thickness of a structural unit is about `10 A^0`.
The two successive structural units are joined together by a link between oxygen Ion of the two silica sheets. The link is due to natural attraction for the cations in the intervening space and due to Vander Waal forces. The negatively charged surface of the silica sheet attract water in the space between two structural units. This results in expansion of the mineral. It may also causes deissociation of the mineral into individual structural units of thickness 10 Angstorm. The soil containing a large amount of the mineral Montmorillonite exhibit high shrinkage and high swelling characteristics. The water in the intervening space can be removed by heating at 200 degree Celsius to 300 degree Celsius.
Montmorillonite minerals have lateral dimension of `0.1 μ` to `0.5 μ` and the thickness of `0.001 μ` to `0.005 μ`. The specific surface is about `800 M^2/(gm)`.
The gibbsite sheet in a Montmorillonite mineral may contain iron or magnesium instead of aluminium. Some of the Silicon atoms in the silica sheet may also have isomorphous substitution. This results in giving the mineral a residual negative charge. It attracts the soil water to form an observed layer, which gives plasticity characteristics to the soil.
ILLITE:
The basic structue of illite is similar to that of Montmorillonite except that there is always substantial replacement of silicones by aluminium in the tetrahedral layers and potassium are between the layers serving to balance the charge resulting from the replacement and to tie the sheet units together. The basic unit is symbolically represented as shown in the figure. The cation bond of illite is weaker than hydrogen bond of kaolinite, but is stronger than the water bond of Montmorillonite. Due to this, the illite crystal has a greater tendency to split into ultimate platelets consisting of gibbsite layer between two silica layers, than that in kaolonite. However, the illite structure does not swell because of moment of water between the sheets, as in the case of Montmorillonite. Illite clay particle may be 50 Angstorm to 500 Angstorm thick and 1000 Angstorm to 5000 angstorm in lateral dimension.The specific surface is about `80 M^2/(gm)`.
3. Explain basic structures of clay minerals.
Clay mineralogy is the science dealing with the structure of clay minerals on microscope, molecular and atomic Scale.The clay minerals are basically composed of tiny crystalline substances of one or more members of a small group of minerals-commonly known as clay minerals. These minerals are evolved mainly from the chemical weathering of certain Rock forming minerals.Chemically these minerals are hydrous aluminosilicates with other metallic ions.
The clay minerals Are divided into three main groups on the basis of their crystalline arrangement as shown in the following table:
Name of minerals and Group | Structural formula |
Kaolin group:
|
|
Montmorrillonite Group:
|
|
Illite Group
|
|
Clay minerals are basically composed of two structural unit:
Tectahedral unit
Octahedral unit
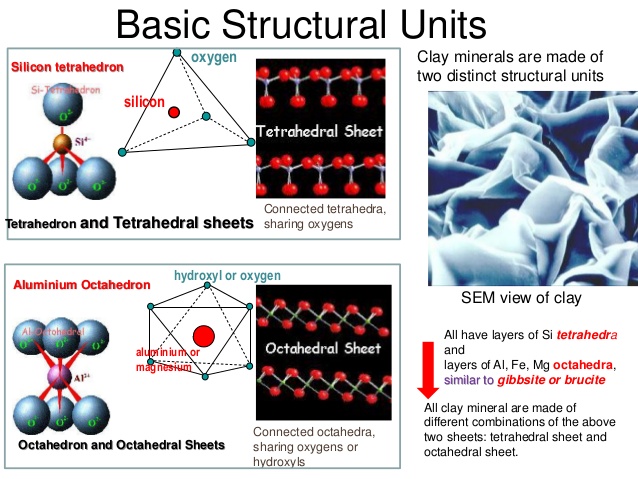
Tetrahedral Units:
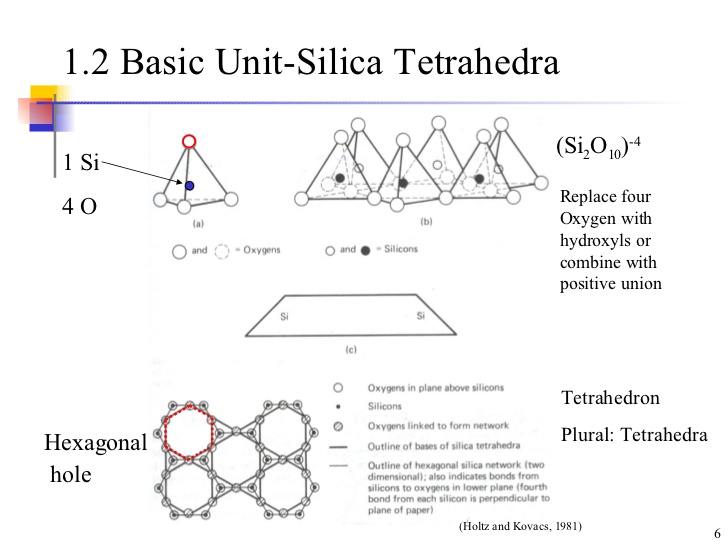
A tetrahedral units consists of a silicon atom surrounded by four oxygen atoms, forming the shape of tetrahedron. Oxygen atom are at the tips of the tetrahedron, whereas the Silicon atom is at its centre. Each of the oxygen ions at the base is common to 2 units. The arrangement is shown in the figure.
Each of the three oxygen ions at the base shares its charges with the adjacent tetrahedral unit. The sharing of charge leaves 3 negative charge at the base per tetrahedral unit and this along with two negative charges at the apex makes a total of five negative charges to balance 4 positive charges of silicon ion . The process of sharing the oxygen Ion at the base with neighbouring units leaves a net charge of -1 per units.
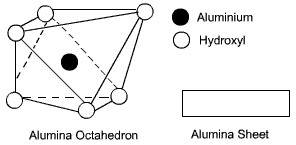
Octahedral units:
An octahedral unit consist of six hydroxyl forming a configuration of an octahedron and having one aluminium atom at the centre. As the aluminium has 3 positive charges, an octahedral unit has three negative charges. Because of net negative charge, an octahedral unit cannot exist in isolation. Several octahedral units combine to form a gibbsite sheet. The sheet is electrically neutral.
Both the structural units can be shown as:
4. Explain various particle force in clay soil.
The forces between soil particles maybe of two types:
- Gravitational force and
- Surfaces force
The gravitational force in a soil particle is proportional to its mass i.e, the larger the particle size, the greater would be the gravitational force. So, they are important in case of coarse grained soils only.
Bonding force or surface force between particles depend upon the surface area of the particles and not upon the volume. The surface area also depends upon the particle size. However, the surface force become more important only when the particle size is small. As the particle size decreases, the effect of surface forces on a particle become becomes more prominent than the gravitational force.
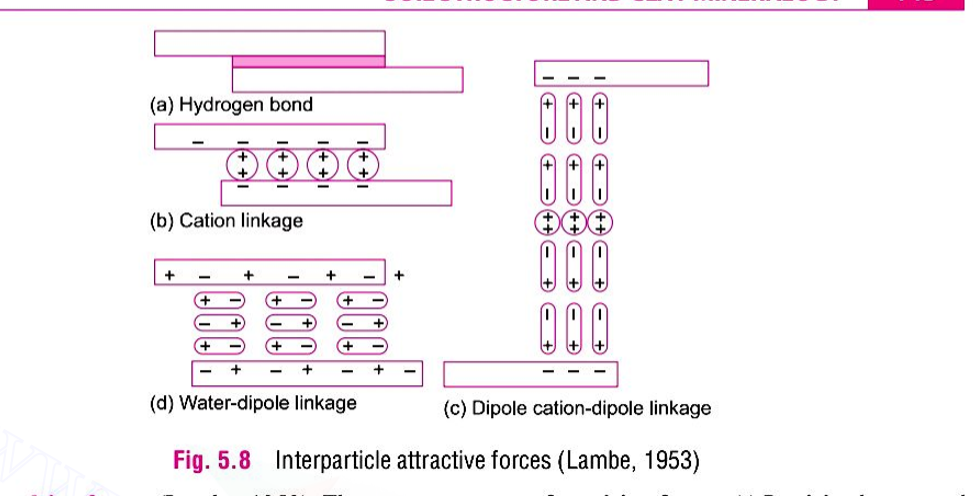
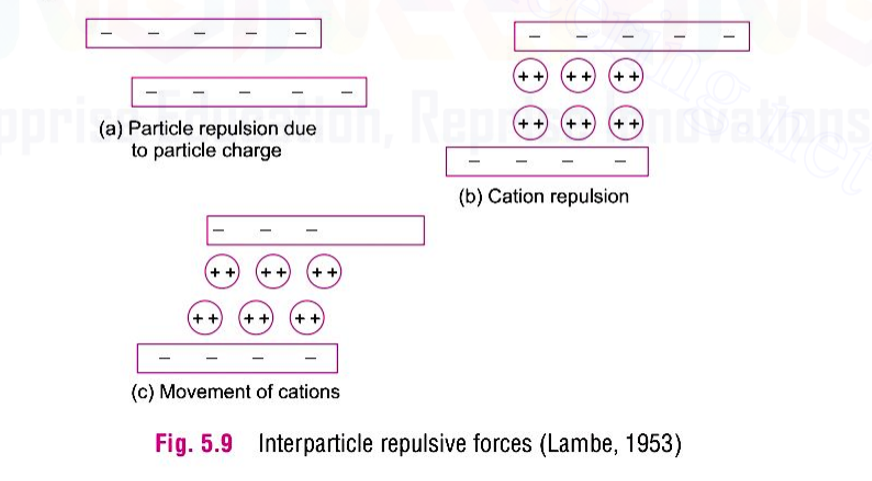
A material in which the surface forces are predominant is called as a colloid. In other words, a colloid is a particle with specific surface so high that its behaviour is influenced by the surface energy than the mass energy. For colloids, the ratio of the surface area to the volume is very large. when the particles are very close to the surface, forces can be attractive or repulsive. Vander Waal force, hydrogen bond, cation linkage, dipole-cation-dipole linkage, water-dipole linkage and ionic bond are the possible mechanisms for attractive forces between particles. The repulsive force between particles is mainly due to similar charges on particle surface. The repulsive force between two adjacent particles become effective when they approach each other and their double layer just overlap.
For a given type of clay in suspension, the net force between the adjacent particle at a given separation is the algebraic sum of of the repulsive and attractive forces acting at that distance. The inter-particle force or potential field decreases with increasing distance from the mineral surface.
If the total potential energy between two particles decreases as they approach each other, the particle will experience attraction and will flocculate. On the other hand, if there is an increase in the total potential energy, there will be repulsion and the particle will disperse. The various factors affecting flocculation or dispersion are electrolyte concentration, temperature, ion balance, PH value, dielectric constant and anion adsorption.
5. Explain various clay particle interaction forces.
The forces between soil particles maybe of two types:
- Gravitational force and
- Surfaces force
The gravitational force in a soil particle is proportional to its mass i.e, the larger the particle size, the greater would be the gravitational force. So, they are important in case of coarse grained soils only.
Bonding force or surface force between particles depend upon the surface area of the particles and not upon the volume. The surface area also depends upon the particle size. However, the surface force become more important only when the particle size is small. As the particle size decreases, the effect of surface forces on a particle become becomes more prominent than the gravitational force.
A material in which the surface forces are predominant is called as a colloid. In other words, a colloid is a particle with specific surface so high that its behaviour is influenced by the surface energy than the mass energy. For colloids, the ratio of the surface area to the volume is very large. when the particles are very close to the surface, forces can be attractive or repulsive. Vander Waal force, hydrogen bond, cation linkage, dipole-cation-dipole linkage, water-dipole linkage and ionic bond are the possible mechanisms for attractive forces between particles. The repulsive force between particles is mainly due to similar charges on particle surface. The repulsive force between two adjacent particles become effective when they approach each other and their double layer just overlap.
For a given type of clay in suspension, the net force between the adjacent particle at a given separation is the algebraic sum of of the repulsive and attractive forces acting at that distance. The inter-particle force or potential field decreases with increasing distance from the mineral surface.
If the total potential energy between two particles decreases as they approach each other, the particle will experience attraction and will flocculate. On the other hand, if there is an increase in the total potential energy, there will be repulsion and the particle will disperse. The various factors affecting flocculation or dispersion are electrolyte concentration, temperature, ion balance, PH value, dielectric constant and anion adsorption.
 Guest
Guest